
The performance of the AIVD glycosylated hemoglobin paired antibody and chromatography uncut sheet is comparable to that of well-known international brands
- Time of issue:2022-09-08
- Views:
(Summary description)According to the International Diabetes Federation, glycated hemoglobin (HbA1c) is the "gold standard" for monitoring diabetes and is crucial for both diagnosing and treating the condition. The glycated hemoglobin level has significant therapeutic relevance for assessing the overall control of blood sugar, identifying treatment-related issues, and directing treatment strategies in the management of diabetes.
The performance of the AIVD glycosylated hemoglobin paired antibody and chromatography uncut sheet is comparable to that of well-known international brands
(Summary description)According to the International Diabetes Federation, glycated hemoglobin (HbA1c) is the "gold standard" for monitoring diabetes and is crucial for both diagnosing and treating the condition. The glycated hemoglobin level has significant therapeutic relevance for assessing the overall control of blood sugar, identifying treatment-related issues, and directing treatment strategies in the management of diabetes.
- Categories:News
- Author:AIVD
- Origin:
- Time of issue:2022-09-08 15:41
- Views:
Hemoglobin in red blood cells and serum carbohydrates combine to form Glycosylated Hemoglobin (GHb). Its composition is determined by the blood glucose concentration and the time that blood glucose and hemoglobin are in contact; it is unaffected by the time of blood collection, the patient's fasting status, the use of insulin, or any other factors. It is created through a slow, continuous, and irreversible glycation reaction. As a result, glycated hemoglobin can accurately reflect a patient's glycemic management during the previous two to three months. Since HbA1c makes up around 70% of GHb and is structurally stable, it is utilized as a monitoring indicator for diabetes management. GHb is made up of HbA1a, HbA1b, and HbA1c.
According to the International Diabetes Federation, glycated hemoglobin (HbA1c) is the "gold standard" for monitoring diabetes and is crucial for both diagnosing and treating the condition. The glycated hemoglobin level has significant therapeutic relevance for assessing the overall control of blood sugar, identifying treatment-related issues, and directing treatment strategies in the management of diabetes.
Product Information
1. Raw Materials
| Product Name | Catalog NO. |
Recommended Usage |
Correlation | Platform |
| Glycated hemoglobin mAb | ABBA1C04 | Coating | R2>0.96 | Immunochromatography Immunoturbidimetric |
| hemoglobin mAb | ABHEMG05 | Labeling |
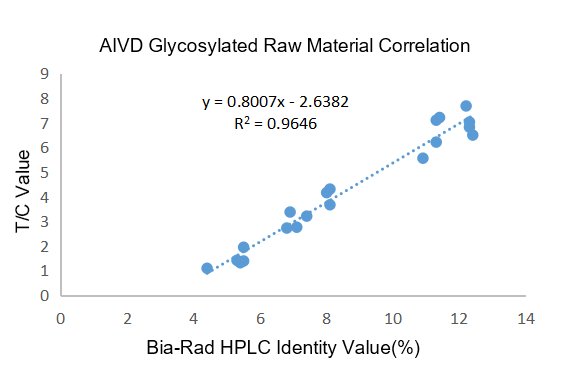
Figure 1. R2 = 0.9646 was used to assess the clinical association between the raw material for the AIVD glycated hemoglobin test and the Bio-Rad glycated hemoglobin HbA1c detection kit.
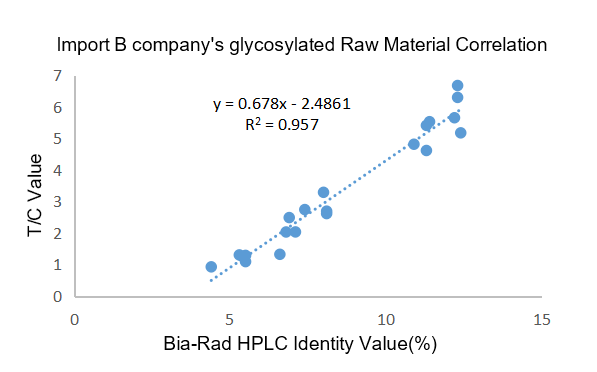
Figure 2. The clinical correlation between the imported raw materials of company B and the Bio-Rad glycosylated hemoglobin HbA1c detection kit (HPLC method) was compared, and R² = 0.9570.
Clinical correlation: The clinical correlation R² = 0.9646 > 0.9570 of AIVD glycated hemoglobin raw materials is better than the imported glycated raw materials of B company.
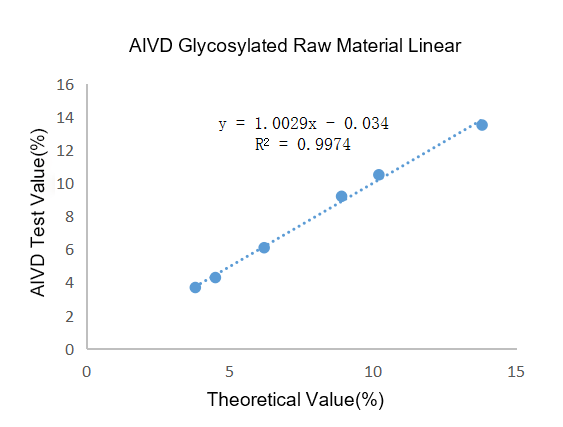
Figure 3 Linearity of AIVD glycosylated raw materials
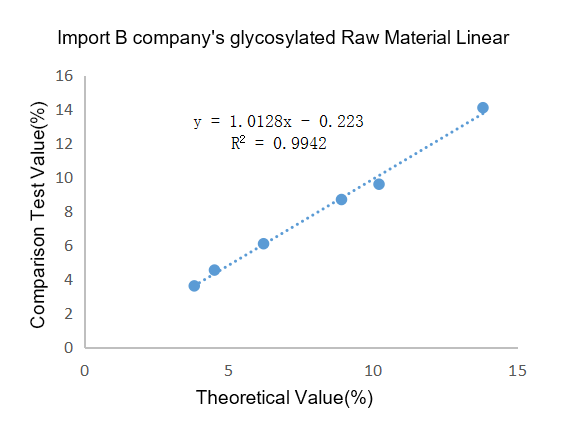
Figure 4 Linearity of imported glycosylated raw materials from Company B
Linearity: The linearity test was carried out on AIVD AI-IF 600 fluorescence immunoassay analyzer. Compared with the theoretical value, R²=0.9974 (Figure 3), and the linear correlation was comparable to the imported glycosylated raw materials of Company B (R²=0.9942, Figure 4). ).
2. Uncut Sheet
| Product Name | Catalog NO. | Linear Range | Correlation | Detection Limit | Specimen |
| Glycosylated Hemoglobin(HbA1c) Test Kit Uncut Sheet (Immunofluorescence Chromatography) | IFHBAD |
4-14% R2>0.99 |
R2>0.96 | 4% | Whole blood |
AIVD is a high-tech company based in China that is committed to provide manufacturers of in vitro diagnostic reagents worldwide comprehensive technical solutions. AIVD can offer complete LDT solutions for IVD firms, medical institutions, and ICL institutions as the first brand of in vitro diagnostic reagent CRO/CDMO service providers! The core team members have extensive backgrounds in the design, manufacture, and registration of products. There are more than 100 people involved, and more than 70% of them work in research and development as graduate, undergraduate, and doctorate students. The current site has a total area of 5,000 square meters, of which 3,000 square meters are dedicated to research and development and 2,000 square meters to production.
| Why Choose AIVD? | ||
 |
 |
|
Technical Support |
Fast Lead Time |
|
 |
 |
|
Bulk Supply |
ISO In-House Production |
|
Related news
Shenzhen AIVD Biotechnology Co. , LTD.
A4 Building 4th Floor / B5 Building C501, China Merchants Bright Technology Park, Fenghuang Street, Guangming District, Shenzhen, Guangdong Province, China
E-mail:
market@aivdbiotech.com
info@aivdbiotech.com
Tel: +86-755-26165742 +86 18543132823
WhatsApp:+86 18543132823
© 2022 Shenzhen AIVD Biotechnology Co.,LTD. 粤ICP备18093805号










